Introduction
NREM2, or light sleep stage 2 (LNREM2), is one of the four sleep stages in humans. Similar to NREM1, it is also a light sleep stage. The immediate difference from NREM1 is that this stage 2 is the transitional sleep stages between SWS and REM sleep. Because of this role, humans spend the most time asleep in this stage, assuming normal sleep conditions.
Despite being a light sleep stage, NREM2 may be a noteworthy pivot in human health; however, it still remains poorly studied and to what extent it is applicable to polyphasic sleeping. This post will serve to enlist multiple possible functions of NREM2 and several correlations with personal well-being. Because there is virtually limitless information on NREM2, we only detail some of its relevant features with polyphasic sleeping.
Preliminary Characteristics of NREM2
First and foremost, NREM2 is the first sleep stage that marks the initiation of actual sleep1; furthermore, it accounts for approximately half of the total sleep duration on monophasic sleep, assuming regular sleep in healthy individuals1. As NREM2 begins, humans start falling asleep, and they lose consciousness of their surrounding environment. This is also groundwork to transition to deeper sleep stages later on.
Other features of NREM2 include, but not limited to:
- After the transition from NREM1, alpha waves retreat and theta waves become observable1.
- NREM2 is the most ideal sleep stage to wake from. Even though it is generally harder to wake from it than from NREM1, there is overall not much grogginess. See Sleep Inertia for more details.
- It is possible to lucid dream during NREM2. However, dream reports are often prosaic and quite rare. In addition, arousal often precedes NREM2 lucid dreams possibly to prepare for lucidity2.
- During sleep restriction, NREM2 is the most affected phase in terms of reduction of time spent in this phase3. This detail is important in that the human body favors more vital sleep stages (SWS and REM). Thus, it suggests that some amount of sleep reduction in polyphasic sleeping is feasible at least short-term and supports the concept of repartitioning.
- NREM2 does not have any sleep pressure; thus, the body will not attempt to make up for lost NREM2 at all. There is, in other words, no NREM2 rebound.
Overall NREM2 Functions
- NREM2 may function as a wakefulness sustainer that boosts alertness4,5. This would explain why polyphasic sleepers need to sleep more frequently than monophasic sleeper as there is some reduction in NREM2.
- However, this function has been challenged as of late.
- There are currently two conflicting hypotheses that attempt to explain why polyphasic sleepers require the scheduling of more frequent sleeps than monophasic sleepers. More information is available here.
- It may be partially responsible for motor memory consolidation6.
Aside from these holistic functions, NREM2 also contains two key components: Sleep spindles and K-complexes. Even though these components are also present in SWS, they are most prominent during NREM2. Both sleep spindles and K-complexes serve different roles in humans, and their behavior and functions will be explored below.
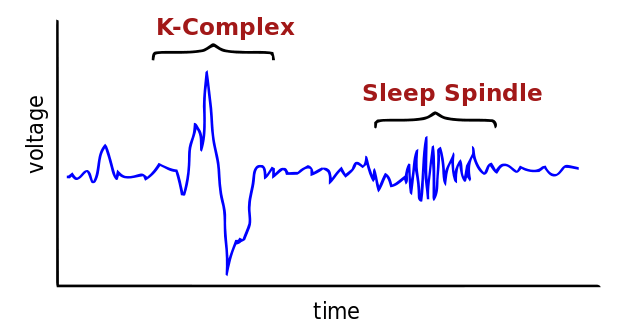
Sleep Spindles
Sleep spindles are waxing and waning rhythmic waves at 7-15 Hz that happen periodically every 3-10 seconds7. For that reason, they are an EEG hallmark of NREM sleep8. A distinction between spindles that occur in NREM2 and SWS is that the NREM2 spindles are visually observable9; meanwhile, SWS spindles can be less distinguishable10.
Roles
Sleep spindles may play a role in the following:
- Memory consolidation11, specifically narrative memory and working memory12
- The development of motor activity13 and sensorimotor functioning12,
- Biomarker for psychiatric disorders such as schizophrenia14–16 and deficits of neural plasticity mechanisms underlying verbal memory alterations17.
- Spindle amplitude, duration and quantity can be biomarkers for Alzheimer’s Disease and Mild Cognitive Impairment (MCI) patients18. This is because said patients have less duration, quantity and lower amplitude of sleep spindles than the healthy elderly group.
- There is an implication that adolescents or growing individuals with higher spindle activity during sleep perform better cognitively19.
- Most importantly, sleep spindles play an important role in the thalamo-cortical network development of the adolescent brain20. Furthermore, sleep spindle quality may aid with more efficient information processing and protect against sleep disturbances. This hallmark of NREM2, therefore, is critical for cognitive development in teenagers.
Classification
However, there are also two different types of spindles: Fast and slow spindles.
- Slow spindles (11-13 Hz) reside over the frontal sites while fast spindles (13-15 Hz) are prevalent at central site18.
- Their difference mostly manifest in their own roles; slow spindles have unknown functions in humans while fast spindles are essential for sleep-dependent memory processing18.
Other notes
- Naps can reliably estimate nocturnal sleep spindle density in health and schizophrenia21. The study concluded that naps contain less spindle activity than the nocturnal core sleep. However, it is unknown if there can be more spindle activity regarding sleep reduction as well as reducing biphasic schedules for instance.
- There is no correlation between NREM2 duration and sleep spindles’ density, amplitude, duration and quantity. This is because Alzheimer’s and MCI patients have more NREM2 while less of those qualities for their own sleep spindles18 than the healthy group. Most notably, they even have less SWS and REM percentage than their healthy elderly counterparts.
- Sleep spindles naturally decrease in density, quantity and duration with age22. Specifically, age-related decline in spindle density and amplitude is more prominent in anterior brain regions; meanwhile, duration is more dominant in posterior regions23. This as a result may explain why the elderly have insomnia and trouble staying asleep.
- During sleep deprivation, spindle activity increases at the beginning of sleep. However, except spindle density, its amplitude and total quantity decrease24. Thus, it may be useful to check spindles’ amplitude and total quantity in adapted polyphasic sleepers to further study their spindles’ integrity.
K-complexes
K-complexes are a salient waveform of sleep and a characteristic feature of NREM2. They also appear in SWS25. They stick out during NREM2 because of their large amplitudes compared with the relatively low amplitude of the background EEG activity; nevertheless, they are less obvious in SWS because of the presence of similarly high amplitude delta waves25.
Roles
- Potential biomarkers for Alzheimer’s Disease and MCI18.
- Periodically syncing and triggering various sleep oscillations26. This acts as a connection to cause loss of consciousness to facilitate sleep.
- Potentially aiding in information processing27.
- In addition, they may contribute to emotional memory similar to sleep spindles and processing of external stimuli during sleep28.
Other notes
- Alzheimer’s and MCI patients have less K-complexes’ amplitude and density than healthy patients18. Similar to sleep spindles, there is no correlation between NREM2 duration and said parameters of K-complexes. More NREM2 does not mean more K-complexes and vice versa.
- Decreased K-complexes activity may lower memory and motor performances17.
- Like sleep spindles, K-complexes’ frequency and amplitude naturally decrease with age (50+ years old)29.
How much NREM2 per day is healthy?
In polyphasic sleeping, NREM2 duration decreases with sleep cut from personal monophasic baseline (except on non-reducing schedules, although a minimal amount of reduction may still take place on these schedules due to the superior placement of the sleep times). However, up to date there has been no research that specifies the minimum requirement for NREM2 duration to optimize health.
Before thinking about removing all NREM2, note that NREM2 cannot be fully eliminated on any polyphasic schedules because of its transitional roles. All polyphasic schedules, no matter how extreme, will always contain some amounts of NREM2.
Further Implications & Applications of NREM2 in Polyphasic Sleep
Therefore, considering all elements thus far, it is necessary for polyphasic sleepers to pay attention to their own sleep spindles and K-complexes’ quality. Take note of the following factors and implications:
- Certain medication, such as flurazepam, can drastically increase spindle activity but significantly reduce K-complexes’ activity and SWS duration30.
- Natural melatonin can help improve spindle quantity20. Thus, implementing and strictly following a consistent dark period setup is highly recommended.
- Learning-dependent tasks also increase spindle density and NREM2 duration as a whole31. While NREM2 duration netted a notable 15% increase, it did not affect total sleep significantly. The tasks here specifically are purely motor-related tasks like simple tracing. SWS and REM duration remain unaffected on the other hand. This result suggests that on polyphasic schedules with at least some core sleep, pure NREM2 naps can in fact be very useful for learning.
- Naps with mostly light sleep are also common on polyphasic schedules. However, most of them locate in the afternoon or later daytime hours. This is when REM sleep’s circadian regulation is not as strong as early daytime hours.
- NREM2’s capacity to increase declarative memory performance in elderly women also tell a story. Those with strong memory performance showed a much higher quantity of sleep spindles and spindle density than those with poorer memory performance32. While potentially controversial, our recommendation is to keep pursuing lifetime learning through different means (e.g, learning new languages) to keep the brain active and engaged.
- For minors, to maximize learning capability, there should not be any reduction in NREM2 duration. This also means that the best opportunity to adopt polyphasic sleep is through non-reducing schedules.
Excessive NREM2
Previously cited research show that excessive NREM2 is dangerous when coupled with low amounts of SWS and REM sleep. The disproportionate sleep stage distribution results in neurodegenerative diseases like Alzheimer’s.
On the opposite spectrum, shorter sleep duration but higher sleep quality suggests higher brain tissue density33. The focus is on sleep depth and the ability to remain asleep for the whole sleep session; however, shorter sleep duration, which may suggest a reduction in NREM2 duration but not REM and SWS, is a benign vision.
Since NREM2 has very noteworthy components that play into its functions, we recommend a reasonable amount of NREM2 on a polyphasic schedule. Currently, the minimum recommendation is a 4-hour total sleep (e.g, Everyman 3) for an average person (8-hour monophasic). In addition, the perpetuated “myths” over the years that light sleep/NREM2 naps are useless are misleading at best, and outright wrong at worst.
Conclusion
While NREM2 is only a light sleep stage, it is far from a wasteful sleep stage in humans. Its two ingredients, sleep spindles and K-complexes, actually play rather important roles in good health long-term. However, NREM2’s behavior on a reducing polyphasic schedule, especially in adapted sleepers, remain unexplained.
Currently, as extrapolated from cited research, NREM2 duration does not seem to be an important decider or barrier for polyphasic sleepers, those who have spent an extended period of time on a reduced sleep total. The future research will be on the permissibility of NREM2 reduction on a polyphasic regime and how sleep spindles and K-complexes may change when humans adapt to split sleep.
Main author: GeneralNguyen & Jelte1234
Olimex Image by: Dakyne
Page last updated: 19 January 2021
Reference
- Ioannides, A. A., Liu, L., Poghosyan, V., & Kostopoulos, G. K. (2017). Using MEG to Understand the Progression of Light Sleep and the Emergence and Functional Roles of Spindles and K-Complexes. Frontiers in Human Neuroscience, 11. doi:10.3389/fnhum.2017.00313. [PubMed]
- Stumbrys, Tadas, and Daniel Erlacher. “Lucid dreaming during NREM sleep: Two case reports.” International Journal of Dream Research 5.2 (2012): 151-155.
- Van D, Maislin G, Mullington J, Dinges D. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117-126. [PubMed]
- MILNER CE, COTE KA. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. Journal of Sleep Research. 2009;18(2):272-281. doi:10.1111/j.1365-2869.2008.00718.x. [PubMed]
- Dhand R, Sohal H. Good sleep, bad sleep! The role of daytime naps in healthy adults. C. 2007;6(1):91-94. doi:10.1097/01.mcp.0000245703.92311.d0. [PubMed]
- Laventure S, Fogel S, Lungu O, et al. NREM2 and Sleep Spindles Are Instrumental to the Consolidation of Motor Sequence Memories. PLoS Biol. 2016;14(3):e1002429. [PubMed]
- Contreras D, Destexhe A, Steriade M. Spindle oscillations during cortical spreading depression in naturally sleeping cats. Neuroscience. 1997;77(4):933-936. [PubMed]
- Achermann P, Borbély A. Temporal evolution of coherence and power in the human sleep electroencephalogram. J Sleep Res. 1998;7 Suppl 1:36-41. [PubMed]
- Loomis A, Harvey E, Hobart G. POTENTIAL RHYTHMS OF THE CEREBRAL CORTEX DURING SLEEP. Science. 1935;81(2111):597-598. [PubMed]
- Steriade M, Nuñez A, Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993;13(8):3266-3283. [PubMed]
- Schabus M, Gruber G, Parapatics S, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27(8):1479-1485. [PubMed]
- Chatburn A, Coussens S, Lushington K, Kennedy D, Baumert M, Kohler M. Sleep Spindle Activity and Cognitive Performance in Healthy Children. Sleep. 2013;36(2):237-243. doi:10.5665/sleep.2380. [PubMed]
- Khazipov R, Sirota A, Leinekugel X, Holmes G, Ben-Ari Y, Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432(7018):758-761. [PubMed]
- Castelnovo A, Graziano B, Ferrarelli F, D’Agostino A. Sleep spindles and slow waves in schizophrenia and related disorders: main findings, challenges and future perspectives. Eur J Neurosci. 2018;48(8):2738-2758. [PubMed]
- Manoach D, Pan J, Purcell S, Stickgold R. Reduced Sleep Spindles in Schizophrenia: A Treatable Endophenotype That Links Risk Genes to Impaired Cognition? Biol Psychiatry. 2016;80(8):599-608. [PubMed]
- Ferrarelli F, Peterson M, Sarasso S, et al. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167(11):1339-1348. [PubMed]
- Rodríguez-Labrada, R., Galicia-Polo, L., Canales-Ochoa, N., Voss, U., Tuin, I., Peña-Acosta, A., … Velázquez-Pérez, L. (2019). Sleep spindles and K-complex activities are decreased in Spinocerebellar Ataxia type 2: relationship to memory and motor performances. Sleep Medicine. doi:10.1016/j.sleep.2019.04.005. [PubMed]
- Liu, S., Pan, J., Tang, K., Lei, Q., He, L., Meng, Y., … Li, Z. (2019). Sleep spindles, K-complexes, limb movements and sleep stage proportions may be biomarkers for amnestic mild cognitive impairment and Alzheimer’s disease. Sleep and Breathing. doi:10.1007/s11325-019-01970-9. [PubMed]
- Reynolds, C. M., Short, M. A., & Gradisar, M. (2018). Sleep spindles and cognitive performance across adolescence: A meta-analytic review. Journal of Adolescence, 66, 55–70. doi:10.1016/j.adolescence.2018.04.003. [PubMed]
- Galván, A. (2019). The Need for Sleep in the Adolescent Brain. Trends in Cognitive Sciences. doi:10.1016/j.tics.2019.11.002. [PubMed]
- Mylonas, D., Tocci, C., Coon, W. G., Baran, B., Kohnke, E. J., Zhu, L., … Manoach, D. S. (2019). Naps reliably estimate nocturnal sleep spindle density in health and schizophrenia. Journal of Sleep Research. doi:10.1111/jsr.12968. [PubMed]
- Nicolas, A., Petit, D., Rompré, S., & Montplaisir, J. (2001). Sleep spindle characteristics in healthy subjects of different age groups. Clinical Neurophysiology, 112(3), 521–527. doi:10.1016/s1388-2457(00)00556-3
- Martin, N., Lafortune, M., Godbout, J., Barakat, M., Robillard, R., Poirier, G., … Carrier, J. (2013). Topography of age-related changes in sleep spindles. Neurobiology of Aging, 34(2), 468–476. doi:10.1016/j.neurobiolaging.2012.05.020
- Dijk, D.-J., Hayes, B., & Czeisler, C. A. (1993). Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain Research, 626(1-2), 190–199. doi:10.1016/0006-8993(93)90579-c. [PubMed]
- Manzar MD, Rajput MM, Zannat W, Pandi-Perumal SR, BaHammam AS, Hussain ME. Spontaneous K-Complex Density in Slow-Wave Sleep. Murillo-Rodriguez E, ed. P. 2016;11(3):e0150929. doi:10.1371/journal.pone.0150929. [PubMed]
- Amzica, F., & Steriade, M. (2002). The functional significance of K-complexes. Sleep Medicine Reviews, 6(2), 139–149. doi:10.1053/smrv.2001.0181. [PubMed]
- Halász P. K-complex, a reactive EEG graph element of NREM sleep: an old chap in a new garment. Sleep Med Rev. 2005;9(5):391-412. [PubMed]
- Caporro M, Haneef Z, Yeh H, et al. Functional MRI of Sleep Spindles and K-complexes. Clin Neurophysiol. 2011;123(2):303-309. [PubMed]
- Wauquier A (October 1993). “Aging and changes in phasic events during sleep”. Physiol. Behav. 54 (4): 803–6. doi:10.1016/0031-9384(93)90095-w. PMID8248360. [PubMed]
- Johnson, L. ., Hanson, K., & Bickford, R. . (1976). Effect of flurazepam on sleep spindles and K-complexes. Electroencephalography and Clinical Neurophysiology, 40(1), 67–77. doi:10.1016/0013-4694(76)90180-2. [PubMed]
- FOGEL, S. M., & SMITH, C. T. (2006). Learning-dependent changes in sleep spindles and Stage 2 sleep. Journal of Sleep Research, 15(3), 250–255. doi:10.1111/j.1365-2869.2006.00522.x. [PubMed]
- Seeck-Hirschner, M., Baier, P. C., Weinhold, S. L., Dittmar, M., Heiermann, S., Aldenhoff, J. B., & Göder, R. (2012). Declarative Memory Performance Is Associated With the Number of Sleep Spindles in Elderly Women. The American Journal of Geriatric Psychiatry, 20(9), 782–788. doi:10.1097/jgp.0b013e31823033da. [PubMed]
- Takeuchi, H., Taki, Y., Nouchi, R., Yokoyama, R., Kotozaki, Y., Nakagawa, S., … Kawashima, R. (2018). Shorter sleep duration and better sleep quality are associated with greater tissue density in the brain. Scientific Reports, 8(1). doi:10.1038/s41598-018-24226-0. [PubMed]