Disclaimer
Cortisol on polyphasic sleep remains a controversial topic. The following blog post contains some conflicting information across various research papers regarding the following topics:
- Cortisol awakening response
- Its roles during and after sleep
- the implications toward its potential applications in polyphasic sleeping.
This blog also tries to explain the convoluted mechanics of cortisol in the body and how it works in polyphasic sleeping. At the time of this blog’s creation, there are 4 research papers with findings on cortisol awakening response to polyphasic sleeping.
Introduction
Cortisol is a primary stress hormone in the system that is responsible for fight-and-flight responses. In addition, it also generates other chemicals when humans react to stress1. A vast array of factors contribute to the rise of cortisol in the body; chronic elevation of cortisol levels is detrimental to human health (e.g, constant stress exposure2,3, overloaded work2, sleep issues3).
With regards to sleep, many simultaneous factors can also affect cortisol levels. Examples include:
- Sleep disturbance3,4
- Early waking4
- Depression5
- Early stress in the day5
- Several other psychiatric disorders like PTSD6.
The goals of this blog post are to:
- List several mechanisms and factors that dictate the levels of cortisol in the human body
- Show how polyphasic sleeping can affect cortisol
- Explain whether polyphasic sleeping can ensure that cortisol levels are stable after adaptation
and
- Postulate whether staying on a polyphasic pattern for an extended period of time (e.g, years) is a healthy lifestyle.
We will also discuss two important markers of cortisol secretion:
- Cortisol levels upon awakening (CAR) from each polyphasic sleep block
- Overall cortisol regulation throughout a day on a polyphasic schedule
Content
- Cortisol Secretion in Monophasic Sleep
- Secretion of Cortisol with Sleep Deprivation
- Studies on Polyphasic Sleep & Cortisol Secretion Patterns
Mechanism of cortisol secretion under nocturnal monophasic condition
Like other hormone secretions, cortisol also goes through a cyclic secretion period marked by the normal 24-hour circadian rhythm. The adrenal cortex secretes cortisol and cortisol is heavily tied to the HPA axis. This axis holds a pivotal role in controlling the stress response system6.
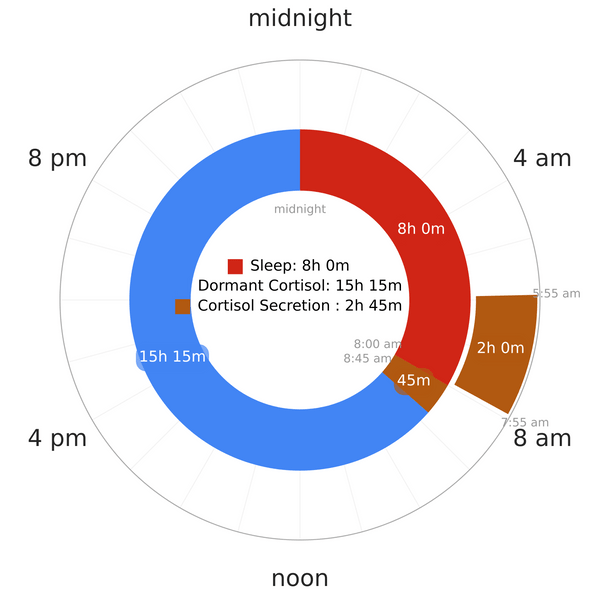
- Under normal, uninterrupted nocturnal sleep conditions, cortisol levels drop to very low during the first half of the night (during SWS).
- It rises during the second half of the night (during REM), which is a few hours before awakening.
- Upon awakening, cortisol concentration remains high for another 30-45 minutes in healthy individuals6,7,8.
- After this brief rise, during the rest of the day, cortisol secretion drops to very low levels9;
- CAR, however, is unrelated to daytime cortisol secretion7.
Regarding sleep architecture, one study claimed that sleep stages do affect CAR because of rhythmic periodicity6.
- Specifically, the difference between a REM wake and an NREM wake (light sleep) affects the magnitude of CAR, owing to the pre-activation of neocortical networks and associated memories6.
- However, given the small sample size of the study, whether only REM sleep causes cortisol to rise, or how sleep stages affect cortisol concentration, especially on a polyphasic sleep regime is inconclusive.
- Another study postulated that cortisol levels are minimal during SWS10. This is consistent with the other conclusions from the studies on cortisol levels during the first half of the night.
Cortisol secretion during sleep deprivation
A lot of studies have analyzed how cortisol levels can change during short or extended sleep deprivation periods, as a result of sleep disturbance and poor sleep quality. Sleep deprivation across these studies were mostly under drastic reduction of nocturnal monophasic core sleep or staying awake for extended periods of time.
There is some similarity between sleep deprivation under these conditions and polyphasic sleep adaptation: partial sleep deprivation; however, the final conclusions remain unclear. This is because adapted polyphasic schedules would be different, especially reducing schedules.
Abnormal morning secretion pattern
In a study of 10 healthy males (aged 19 to 27):
- The lowest point of cortisol secretion is from 23:00 to 03:00.
- The highest point (peak) is from 07:00 to 11:0010.
- This inconsistent data from regular cortisol secretion pattern with sufficient nocturnal sleep (CAR only persists for 30-45 minutes upon awakening) results from 40 hours staying awake.
- Afterwards, there was an unlimited nocturnal sleep session. There were no alarms and recovery was under laboratory environment.
This implies that one recovery night sleep may not be sufficient to re-stabilize cortisol levels. However, more implications about cortisol secretion during sleep deprivation shows a lot of interesting findings.
Atypical evening secretion pattern
It is predictable that CAR would steeply increase during waking hours after at least one night of partial sleep deprivation. However, several studies have shown otherwise.
- Cortisol levels are “blunted” upon awakening11-13.
- Surprisingly, there is a delay in cortisol rise and instead it only sharply increases in the evening1,11.
There is also inconsistent data on CAR peaks across studies on sleep deprivation. This is because of lack of understanding and explanation of numerous factors:
- Timing of awakening from sleep,
- Sleep stages,
- Lack of sampling before and after awakening,
- Insufficient sample sizes
Extended sleep deprivation periods also keep cortisol levels elevated during waking hours until a negative feedback (upregulation) within the HPA system occurs; this happens when sleep initiates14.
Concerns on polyphasic sleep
Regardless, the changes in cortisol secretion pattern under sleep deprivation (akin to polyphasic adaptation) is a concerning matter. There are different polyphasic schedules with very different sleep-wake hours; this is despite the standard variants focus on as much nocturnal sleep as possible.
It is possible that cortisol secretion patterns during sleep deprivation from polyphasic adaptation are messy and unpredictable. However, currently, no data from the community directly measures CAR intensity as well as daily cortisol peak and trough concentrations.
Cortisol regulation in four polyphasic-related studies
So far, there have been few studies between CAR and polyphasic sleeping. This section will analyze four polyphasic-related studies and attempt to correlate how these polyphasic sleep experiments alter CAR activation and cortisol secretion patterns.
As usual, the explanations from these studies do not provide enough evidence to apply across all polyphasic schedules and all polyphasic sleepers.
STUDY 1: INVESTIGATION OF NAPPING BEHAVIOR
PART 1: Non-reducing Siesta (Afternoon nap)
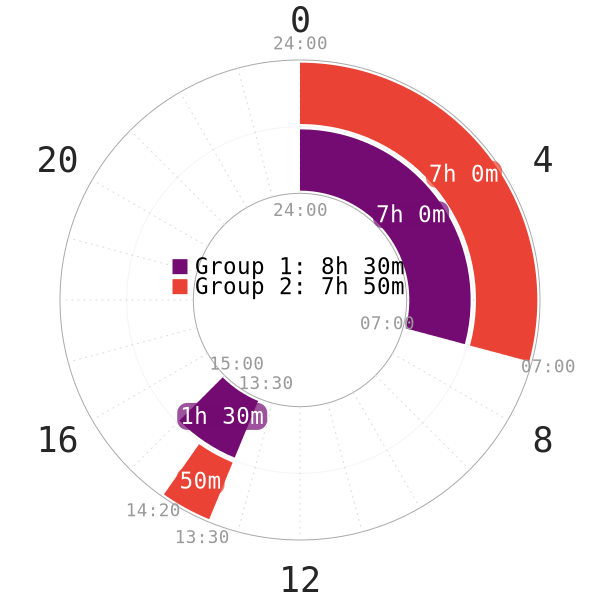
Introduction
The first part of the study explores whether CAR is present after a nap; whether nap duration, timing of naps in the day and sleep architecture are involved in activating CAR5. However, the following aspects are noteworthy:
- None of the subjects were habitual nappers before the experiment
- All of the subjects had a normal monophasic sleep schedule (bedtime starts between 22:00 and 24:00 and wake time starts between 06:00 and 10:00)
- These participants were healthy and free of any sleep, psychological disorders or any head-related injuries.
- All subjects did not consume any medications or substances.
- All of the subjects started lying in bed in preparation for the nap at 13:30.
- The data sampling ended at 15:30 to record all subjects who managed to sleep at least 50 minutes.
NOTE: The napchart in Figure 1 is only an illustration of the subjects’ sleep patterns. Start of night sleep and wake of morning sleep are likely to vary by a decent margin. There is no pre-existing sleep deprivation period or any nocturnal sleep restriction before the experiment. Thus, Siesta-extended (non-reducing type) is likely the schedule of choice.
This naming is to make it consistent with the nomenclature of polyphasic schedules on this website.
Interpretation of results
Results from this study demonstrated that there was a period of nCAR upon the 90-minute naps, but not 50-minute naps. nCAR is cortisol increase as a response to napping.
- One possible explanation is that the 50-minute naps are too short to induce an nCAR.
- In contrast, 90 minutes, which is a “core” in the polyphasic community, is long enough. It likely contains all vital sleep stages.
- nCAR in the study exhibited similar behavior to CAR from nocturnal sleep. There was a 30-45 minute rise in cortisol levels upon awakening.
- However, nCAR’s size is twice as small as CAR size from nocturnal sleep. This is no surprise given nocturnal sleep duration is a lot longer than a nap.
- Interestingly enough, cortisol secretion levels from 50-minute naps were drastically higher than those from 90-minute naps.
- The study explained that 90-minute “naps” contained all sleep stages (higher percentage of REM/SWS).
- They were long enough to suppress cortisol secretion during sleep, which in return caused low cortisol levels upon awakening.
- 50-minute naps by their structure contained a higher percentage of light sleep and only a small amount of SWS. Thus, they did not last long enough to fully suppress cortisol levels during sleep.
PART 2: Non-reduced Segmented (Morning nap)
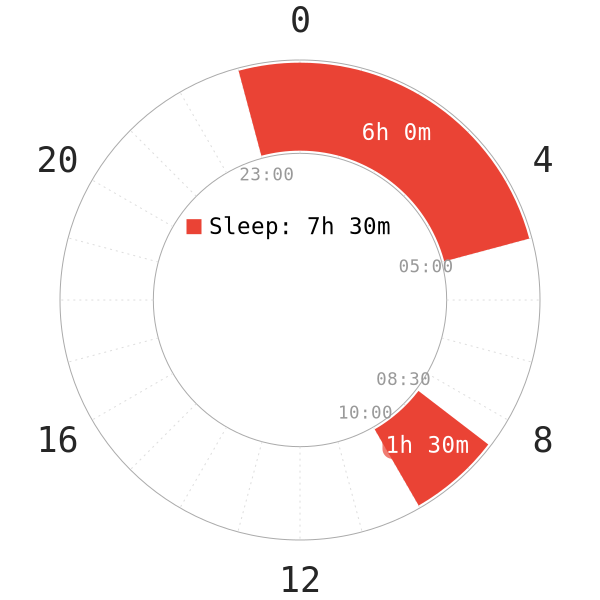
Introduction
The second part of the study delves into the effects of a 90m sleep around dawn (REM peak) on nCAR. Similar to the first part of the study, the following points are noteworthy:
- None of the subjects were habitual nappers before the experiment.
- All of the subjects had a normal monophasic sleep schedule. The bedtime starts between 22:00 and 24:00 and wake time starts between 06:00 and 10:00.
- The participants were healthy and free of any sleep, psychological disorders or any head-related injuries.
- Everyone did not consume any medications or substances.
- All subjects started getting into bed at 23:00. They remained asleep until 05:00 (6-hour core sleep).
- Upon the first core, all subjects can read and watch TV until 08:30, when they started the second sleep. Same as the subjects in part 1, a 2-hour timespan was dedicated to data sampling (ended at 10:30). This went on until these subjects slept for 90 minutes.
- All subjects took either Zolpidem (a sedative that increases SWS amount) or a placebo before lights out.
- 5-10 days later, all subjects returned for the second test session. Those who received a placebo now received Zolpidem and vice versa.
Interpretation of results
- The results showed that the 90m sleep around dawn also consistently resulted in an nCAR.
- The sleep duration around dawn is usually REM-heavy (especially once adaptation is complete).
- The subjects did manage to get more REM than SWS in the second core, either with or without Zolpidem administration.
- Researchers concluded that timing of sleep and sleep duration play a role in generating nCAR and CAR instances. This observation is consistent with another study9.
Takeaways
- Sleep architecture (from previous findings) shows that NREM1 and NREM2 potentially increases HPA axis’ activity during sleep. This would result in a higher cortisol levels upon awakening.
- Sleep stages, circadian timing and sleep duration are considerable factors. This is because all of them are contributors to creating nCAR(s) and increasing cortisol levels.
- Full-cycle sleeps seem to produce CAR and nCAR(s) because of the length, but not smaller naps (50 minutes and shorter).
However, it also currently inconclusive whether a fully repartitioned short nap (e.g, 20m REM nap around dawn) is equivalent to 90-minute variants or not. More long-term studies on sleep repartitioning of more extreme polyphasic schedules are necessary to make further correlations about how these sleep regimes regulate cortisol.
STUDY 2: UBERMAN SLEEP EXPERIMENT
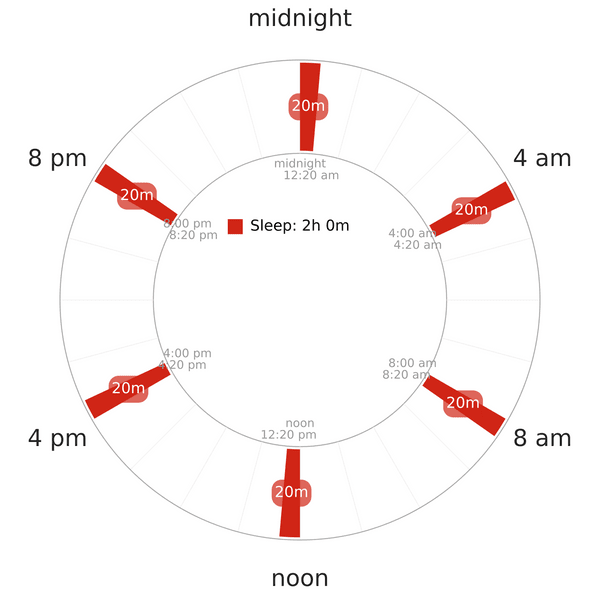
Introduction & Results
This is one of the few studies on an extreme polyphasic schedule and its related endocrine and cognitive effects on human. Another relevant assessment is the mentioning of cortisol level before and after the experiment. The study consists of the following important points15:
- 8 healthy young males (age 21-28) changed their monophasic lifestyle to Uberman sleep.
- The schedule structure is similar to Uberman’s (a 20-minute nap every 4 hours).
- There were extensive 24-hour physio, psychiatric health screening and blood sampling every 30 minutes before and after the study.
- The study lasted for 5 weeks.
- 7 subjects dropped out of the study after 3 weeks mainly due to social life constraints (but not sleep deprivation).
- The remaining subject did not show any cognitive impairment after 5 weeks.
- Cortisol release pattern did not differ before and after 5 weeks.
NOTE: The napchart above illustrates the Uberman schedule; it does not reflect the accurate sleep times of the subjects in the study. Unfortunately, all information about this study’s results and analysis is not available yet. No specific data on how cortisol level changed throughout the course of 5 weeks is accessible.
Only the Abstract section is viewable up to date. Thus, most of the remaining explanations and correlations are speculative.
SPECULATIONS & IMPLICATIONS
Comparison between Monophasic & Uberman sleep
- None of the subjects had abnormal genetic mutations (e.g, DEC2 gene) that enable them to sleep much less than average humans.
- These subjects also most likely maintained some consistent nocturnal monophasic pattern before the experiment. As a result, their cortisol regulation is presumably normal; low in the first hours of sleep, gradual rise close to awakening and remaining high for 30-45 minutes upon awakening.
- There was no mention of any oversleeping, so the assumption is that they possibly made it through at least 3 weeks (for 7 subjects) and 5 weeks (for 1 subject) without oversleeping. This is a reasonable expectation because all subjects were under human supervision in laboratory conditions. They also had activities to do, so they could sustain a 22-hour wake with certain personal motivation (e.g, gaining more waking hours). As such, adaptation theoretically would progress until an equilibrium point between SWS, REM and light sleep.
- There were no exact numbers for EEG measurements of sleep stage percentage, so it is not conclusive if the remaining subject fully adapted to the schedule.
Possible Cortisol Secretion Patterns on Uberman sleep
- Due to the extreme nature of Uberman, 5 weeks might not be sufficient to conclude the the adaptation phase (at least for normal, average humans).
- Anecdotally, one long-term Uberman sleeper in the community pointed out that oversleeping could still happen even after 2 years on the schedule.
- Crashes can happen without any specific reasons, not because of sickness.
- See Uberman for information about this case. However, adaptation duration usually varies from person to person.
- Since the last subject did not show any cognitive impairments and no change in the stress regulation system, it is likely that the subject managed to adapt to Uberman short term. There probably was an equilibrium of vital sleep stages; their mental state possibly became stable once sleep deprivation reached a minimal value and a plateau.
- There was no change in cortisol secretion pattern before and after the study.
- This suggests that after 5 weeks on Uberman, cortisol levels are predictably low in the evening and the first half of the night. This is during the midnight or late evening nap, anywhere between 8 PM and midnight.
- Cortisol levels are high in the morning (upon awakening from the dawn nap and persisted for some time after awakening). Otherwise, cortisol levels remain consistently low during daytime hours. This would match with their cortisol secretion pattern on monophasic sleep.
- Their stress-controlling system (HPA axis, glucocorticoid and cortisol) is probably healthy. It may be even sustainable for an extended period of time on an extreme sleep restriction regime.
- However, during the adaptation period, it is possible that the cortisol levels and CAR activations were chaotic. Regardless, the exact details of CAR and cortisol levels during the day could not be concluded because of no accessible data.
REM & SWS naps
- Whether REM naps or SWS naps determine CAR incidents in the Uberman schedule is unknown. Hypothetically, if the cortisol secretion pattern did not change after 5 weeks, it is possible that a nap full of vital sleep stage percentage is enough to suppress cortisol during sleep, resulting in a weaker CAR upon awakening.
- Anecdotally, a REM nap feels like a lot of time has passed, so it is possible that naps full of REM can replace hours of regular nocturnal monophasic sleep.
- Based on the Uberman schedule’s structure, the morning nap full of REM was likely to generate a CAR.
Adapted polyphasic schedules
- It is possible that adapted polyphasic schedules (with at least a core sleep of 90 minutes minimum) eventually result in a stable CAR pattern, although how cortisol level is changed and to what extent are unknown. This is in line with a stable mood and good ability to handle stressful situations in anecdotally adapted individuals, because sleep deprivation symptoms are gone and vital sleep stages are ensured.
- Whether all CAR results are consistent across different polyphasic patterns (Dual Core, Everyman, etc.) are yet to be confirmed. Theoretically, the degree of sleep reduction, timing of naps (from the first study), circadian cues (e.g, dark period implementation, light usage at night), sleep genes are some of the most important factors that potentially affect cortisol secretions and CAR instances.
STUDY 3: INVESTIGATION OF NAPPING BEHAVIOR (NON-REDUCING SIESTA)
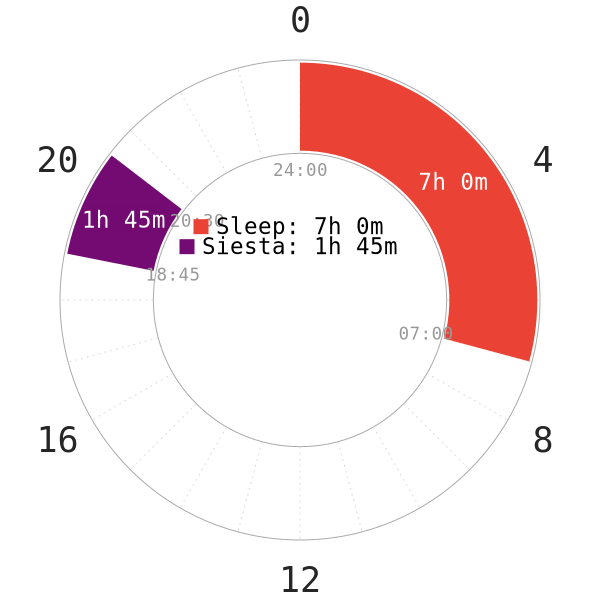
NOTE: Since there was no mention on how much sleep the students got each night, the nocturnal sleep in the napchart is only an illustration.
Introduction
A few pointers to note for this study16:
- The students took the “nap” only on 2 consecutive days in the week.
- None of them were habitual nappers before the experiment.
- There was no mention of sleep restriction; therefore, it is likely that the students were not under any severe sleep deprivation (or not at all) before nap time.
- 3 of the subjects were habitual smokers.
- No smoking, eating or drinking after 1 hour of awakening for salivary sampling.
- No brushing teeth to avoid minor blood contamination from cavity.
Results, Discussion & Implications
- Lack of CAR from students’ naps was consistent across the samples.
- Waking up as an independent process is not sufficient for an increase in CAR.
- Timing of the nap and circadian cues play a role in generating CAR instances. From other studies, earlier naps during the day (early afternoon, noon and morning) resulted in consistent CARs4.
- Due to the siesta’s timing, this seems to clash with the Forbidden Zone of Sleep (FZS)17.
- The students had a regular sleep-wake schedule; thus their FZS hours likely coincide with these late evening hours.
- Since no sleep deprivation was detectable (no mention of whether they skipped night sleep to nap at these hours), the students did not sleep deeply. As a result, their sleep was lighter with less vital sleep stages at these hours.
- The students also only started sleeping at these hours for 2 days. Longer periods of entrainment with this sleep block might yield different results. However, more research on FZS and cortisol is mandatory for further conclusions.
- Despite having a core sleep of 105 minutes, the study mentioned that this sleep duration might not be enough to generate an nCAR.
STUDY 4: MULTIPLE-NAP SCHEDULE, NON-REDUCING TYPE
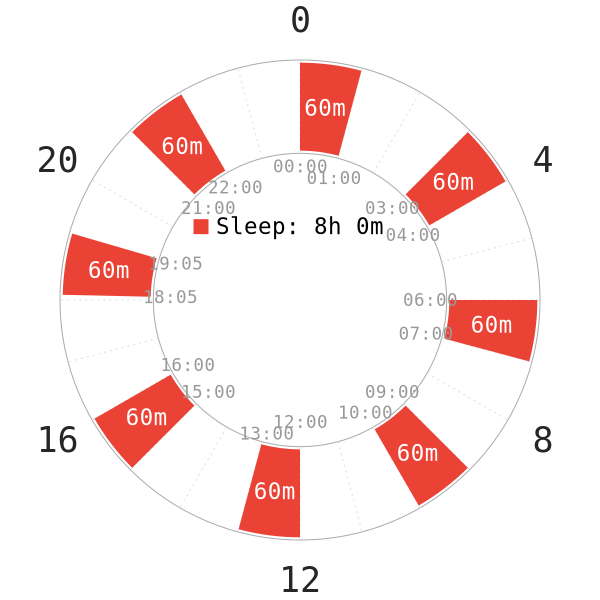
Introduction
The following details regarding methods and preliminary information are to be noted in this study18:
- 7 healthy young adult subjects (6 men, 1 woman aged 23-40)
- All subjects had a normal sleep schedule, 7-8 hours of nocturnal monophasic sleep.
- There was a recovery week before the experiment of the 3-hour day schedule. This was when they would sleep for 1 hour and stay awake for 2 hours.
- The experiment on this polyphasic schedule lasted for 10 full days.
- If the subjects were asleep when lights were on, they would be awakened.
- There was a recovery period of 1 week after this polyphasic schedule.
Results, Discussion & Implications
- Body temperature and plasma cortisol concentrations resemble those of regular 24 hour monophasic sleep pattern throughout the whole duration of the experiment.
- This insinuates the resilience of the normal 24 hour circadian rhythm;
- Cortisol secretion pattern would not change if the study continued. This is there were no foreseeable or identifiable factors that could result in a change.
- 10 days might not be sufficient to change the pattern of cortisol secretion. This is appropriate because adaptations to polyphasic schedules with similar structure (sleeping in smaller sleep blocks) requires full sleep repartitioning. Thus, this observation remains inconclusive.
- All subjects were sleep deprived.
- NREM sleep (light sleep and SWS) remained mostly intact while there was a penalty for REM sleep. This suggests that an adaptation period was in progress.
- The highest amount of CAR reportedly occurred after sleep periods with maximal REM sleep.
- Cortisol secretion did not happen in the evening hours, 21:00-22:00. All subjects did not fall asleep or only managed to get little sleep here. However, the difference between SWS and REM distribution during sleep could not determine the effect on CAR. Rather, the proposed reasoning states that the increase in CAR intensity is because of a “rude awakening”; this is a stressful transition from sleep to wakeful states.
Implications from four studies
The four studies present more questions than answers about how certain factors affect CAR. Furthermore, the most dominant factor in creating CAR instances is still unclear.
- The first and the third studies both tested a long sleep duration (at least 1 full cycle, 90 minutes long); yet results were contradictory. However, the conclusions from both studies were consistent.
- Timing of sleep and circadian rhythm can affect CAR’s creation, even if sleep duration is the same.
- These two studies along with the fourth study shared a common ground: sleep blocks with a high REM percentage often result in a CAR instance.
- The process of waking up and sleep stage composition are interesting topics for further exploration; they appear to be suspecting factors for CAR’s occurrences.
- The final verdict remains unclear. It is not known if a specific sleep stage at different times of the day has to do with CAR.
- The second study on Uberman sleep sparked some insights into whether a polyphasic adaptation is possible. The results seem to support the notion that a successful polyphasic adaptation can stabilize cortisol levels.
Conclusion
There have been a lot of contradictory findings on cortisol secretion and CAR mechanisms. Several factors supposedly affect cortisol secretion patterns and cortisol production concentration. The general consensus is that cortisol levels rise prior to awakening in the morning and are generally low during the day; however, this seems to vary on polyphasic patterns during the adaptation period.
Speculatively, successful polyphasic adaptation would result in a consistent secretion pattern of cortisol and CAR instances; nonetheless, the current sample sizes from the polyphasic sleep studies are too small to draw a broad conclusion.
Main author: GeneralNguyen
Page last updated: 8 April 2021
Reference
- Leproult, R., et al. “Sleep Loss Results in an Elevation of Cortisol Levels the next Evening.” Sleep. 1997;20(10):865–870. [PubMed]
- Schlotz, Wolff, et al. “Perceived Work Overload and Chronic Worrying Predict Weekend-Weekday Differences in the Cortisol Awakening Response.” Psychosomatic Medicine. 2004;66(2):207–214. [PubMed]
- “Chronic Stress Puts Your Health at Risk.” Mayo Clinic, 2016, www.mayoclinic.org/healthy-lifestyle/stress-management/in-depth/stress/art-20046037. Accessed 3 March. 2020.
- Williams, Emily, et al. “The Impact of Time of Waking and Concurrent Subjective Stress on the Cortisol Response to Awakening.” Psychoneuroendocrinology. 2005;30(2):139–148. [PubMed]
- Devine, Jaime K., and Jutta M. Wolf. “Determinants of Cortisol Awakening Responses to Naps and Nighttime Sleep.” Psychoneuroendocrinology. 2016;63:128–134. doi:10.1016/j.psyneuen.2015.09.016. [PubMed]
- Wilhelm, Ines, et al. “Is the Cortisol Awakening Rise a Response to Awakening?” Psychoneuroendocrinology. 2007;32(4)358–366. doi:10.1016/j.psyneuen.2007.01.008. [PubMed]
- Chida, Yoichi, and Andrew Steptoe. “Cortisol Awakening Response and Psychosocial Factors: A Systematic Review and Meta-Analysis.” Biological Psychology. 2009;80(3):265–278. doi:10.1016/j.biopsycho.2008.10.004. [PubMed]
- Stalder, Tobias, et al. “Assessment of the Cortisol Awakening Response: Expert Consensus Guidelines.” Psychoneuroendocrinology. 2016;63(1):414–432. doi:10.1016/j.psyneuen.2015.10.010. [PubMed]
- Späth-Schwalbe, E., et al. “Nocturnal Adrenocorticotropin and Cortisol Secretion Depends on Sleep Duration and Decreases in Association with Spontaneous Awakening in the Morning.” The Journal of Clinical Endocrinology and Metabolism. 1992;75(6):1431–1435. doi:10.1210/jcem.75.6.1334495. [PubMed]
- Davidson, J R, et al. “Growth Hormone and Cortisol Secretion in Relation to Sleep and Wakefulness.” Journal of Psychiatry & Neuroscience : JPN. 1991;16(2):96–102. [PubMed]
- Kudielka, Brigitte M., et al. “Acute HPA Axis Responses, Heart Rate, and Mood Changes to Psychosocial Stress (TSST) in Humans at Different Times of Day.” Psychoneuroendocrinology. 2004;29(8): 983–992. doi:10.1016/j.psyneuen.2003.08.009. [PubMed]
- Omisade, Antonina, et al. “Impact of Acute Sleep Restriction on Cortisol and Leptin Levels in Young Women.” Physiology & Behavior. 2010;99(5):651–656. doi:10.1016/j.physbeh.2010.01.028. [PubMed]
- SCHUSSLER, P, et al. “Nocturnal Ghrelin, ACTH, GH and Cortisol Secretion after Sleep Deprivation in Humans.” Psychoneuroendocrinology. 2006;31(8):915–923. doi:10.1016/j.psyneuen.2006.05.002. [PubMed]
- Späth-Schwalbe, E., et al. “Sleep Disruption Alters Nocturnal ACTH and Cortisol Secretory Patterns.” Biological Psychiatry. 1991;29(6):575–584. doi:10.1016/0006-3223(91)90093-2. [PubMed]
- Mahmoud Rak, et al. Endocrine and Cognitive Effects of a Radically Polyphasic Sleep Schedule. 2013, www.semanticscholar.org/paper/Endocrine-and-cognitive-effects-of-a-radically-Rak-Kunath/b850189afb915e416bcdf9bd58acb0c9ad1e1015. Accessed 10 Mar. 2020.
- Federenko, I. “Free Cortisol Awakening Responses Are Influenced by Awakening Time.” Psychoneuroendocrinology. 2004;29(2):174–184. doi:10.1016/s0306-4530(03)00021-0. [PubMed]
- Stampi, Claudio. Why We Nap : Evolution, Chronobiology, and Functions of Polyphasic and Ultrashort Sleep. Birkhauser, 2014.
- Weitzman, E. D., et al. “Effects of a Prolonged 3-Hour Sleep-Wake Cycle on Sleep Stages, Plasma Cortisol, Growth Hormone and Body Temperature in Man.” The Journal of Clinical Endocrinology and Metabolism. 1974;38(6):1018–1030. doi:10.1210/jcem-38-6-1018. [PubMed]
- Leproult, R., et al. “Transition from Dim to Bright Light in the Morning Induces an Immediate Elevation of Cortisol Levels.” The Journal of Clinical Endocrinology and Metabolism. 2001;86(1):151–157. doi:10.1210/jcem.86.1.7102. [PubMed]

One thought on “Cortisol Secretion Pattern in Polyphasic Sleeping”